
Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange - ScienceDirect

Degradation mechanism and toxicity reduction of methyl orange dye by a newly isolated bacterium Pseudomonas aeruginosa MZ520730 - ScienceDirect

Effective Catalytic Reduction of Methyl Orange Catalyzed by the Encapsulated Random Alloy Palladium‐Gold Nanoparticles Dendrimer. - Ilunga - 2017 - ChemistrySelect - Wiley Online Library
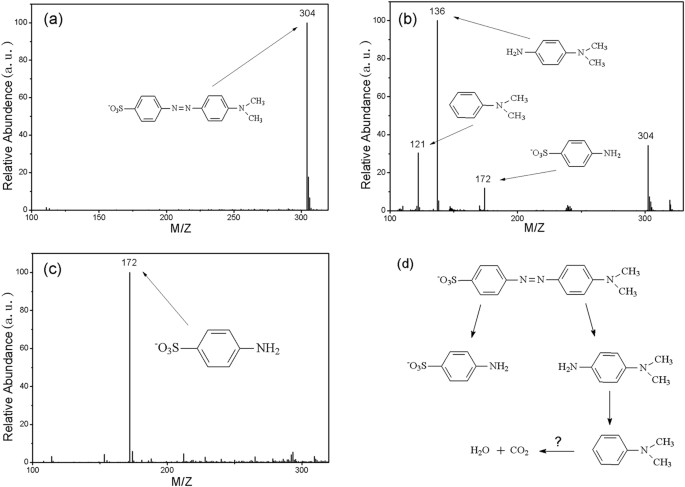
A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders | Scientific Reports

Effective Catalytic Reduction of Methyl Orange Catalyzed by the Encapsulated Random Alloy Palladium‐Gold Nanoparticles Dendrimer. - Ilunga - 2017 - ChemistrySelect - Wiley Online Library
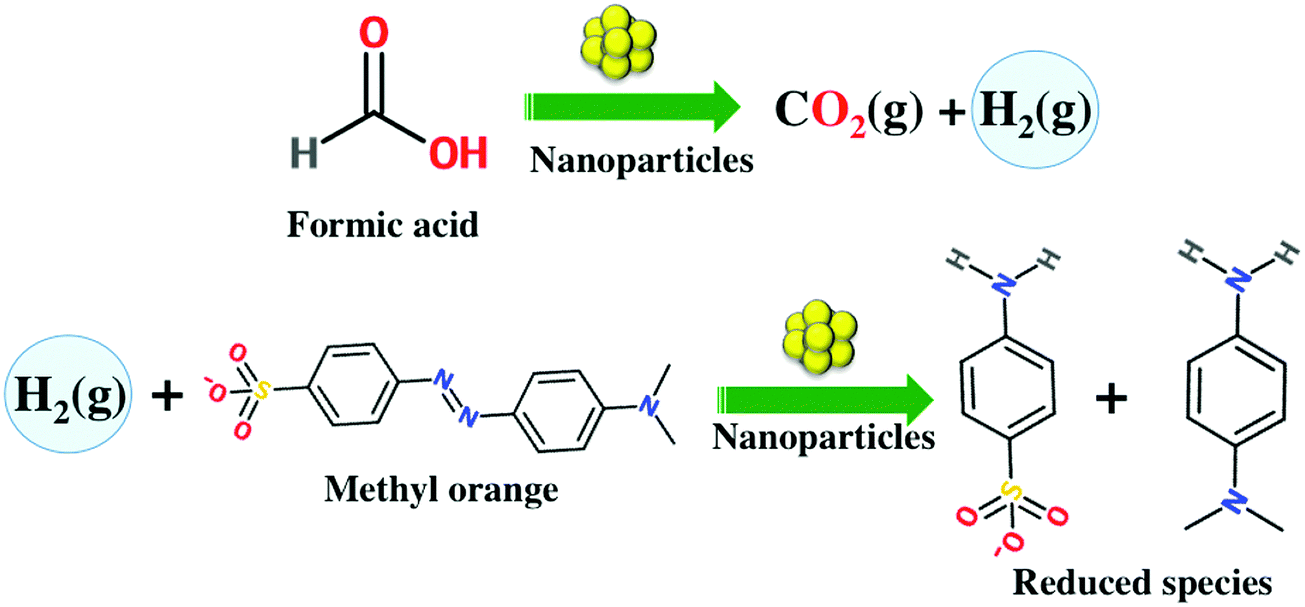
Tannic acid and palladium-modified magnetite nanoparticles for catalytic degradation of methyl orange - American Chemical Society

Degradation mechanism of Methyl Orange by electrochemical process on RuO(x)-PdO/Ti electrode. | Semantic Scholar

Degradation mechanism of Methyl Orange by electrochemical process on RuO(x)-PdO/Ti electrode. | Semantic Scholar

Molecules | Free Full-Text | Synergistic Promotion of Photocatalytic Degradation of Methyl Orange by Fluorine- and Silicon-Doped TiO2/AC Composite Material

Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways - ScienceDirect

Polyaniline Supported Palladium Catalyzed Reductive Degradation of Dyes Under Mild Condition | Bentham Science
IJMS | Free Full-Text | Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation

Rapid Photocatalytic Decolorization of Methyl Orange under Visible Light Using VS4/Carbon Powder Nanocomposites | ACS Sustainable Chemistry & Engineering

Palladium nanoparticles supported on ionic liquid and glucosamine-modified magnetic iron oxide as a catalyst in reduction reactions | SpringerLink

The specialized twin-solution method for selective Pd(II) ions determination and methyl orange removal - ScienceDirect
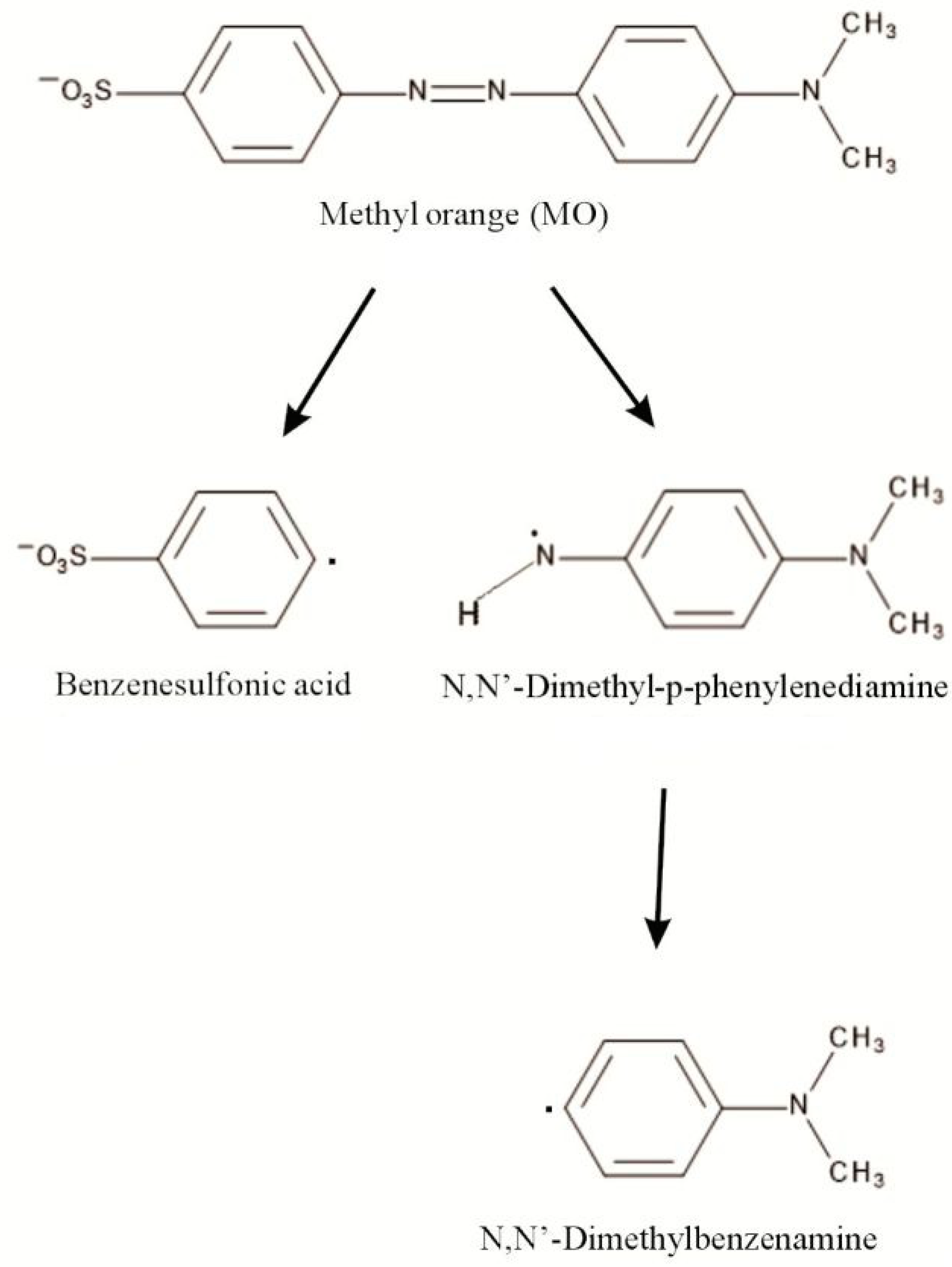
Green synthesis of palladium nanoparticles and investigation of their catalytic activity for methylene blue, methyl orange and rhodamine B degradation by sodium borohydride | SpringerLink

Enhanced Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution by Alkali-Activated Multiwalled Carbon Nanotubes | ACS Applied Materials & Interfaces
Green synthesis of gold, silver, platinum, and palladium nanoparticles reduced and stabilized by sodium rhodizonate and their catalytic reduction of 4 ... - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C8NJ01223G

Acceleration of biotic decolorization and partial mineralization of methyl orange by a photo-assisted n-type semiconductor - ScienceDirect
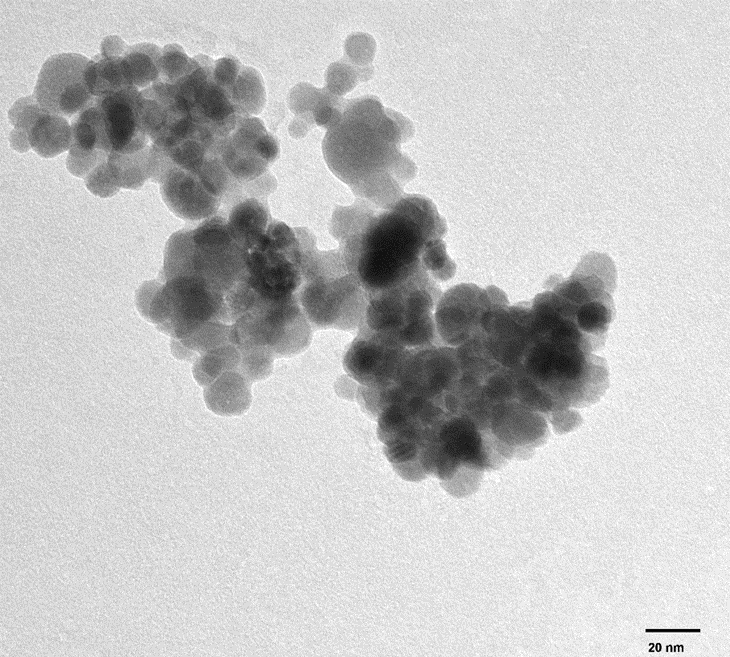
Tannic acid and palladium-modified magnetite nanoparticles for catalytic degradation of methyl orange - American Chemical Society
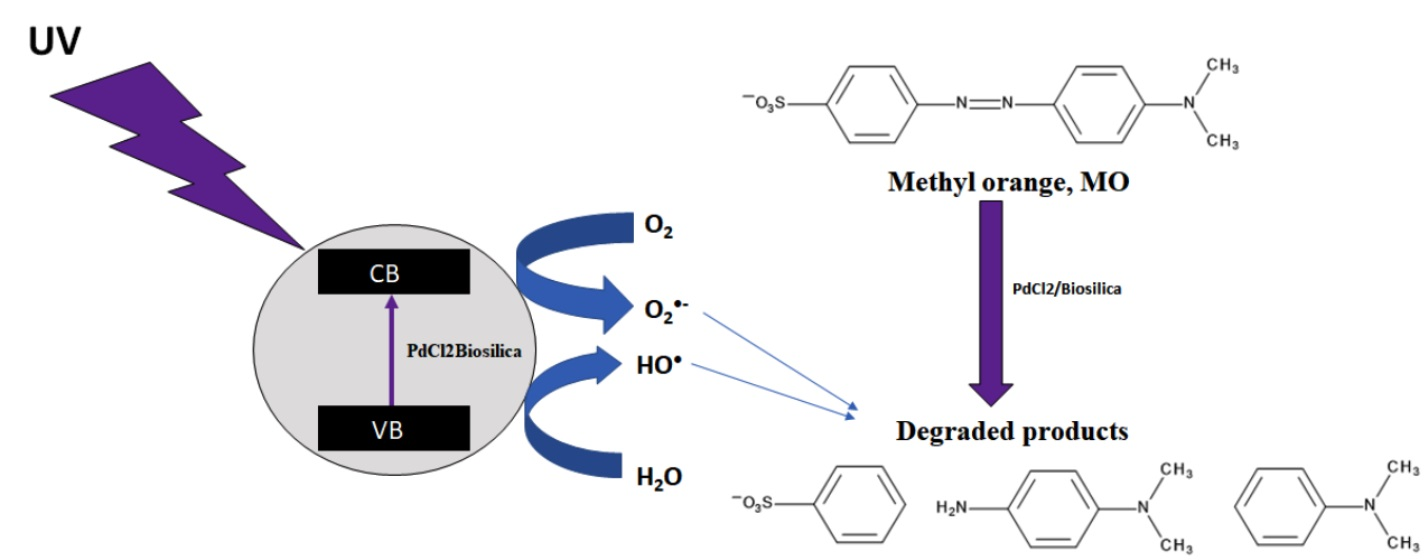
IJMS | Free Full-Text | Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation
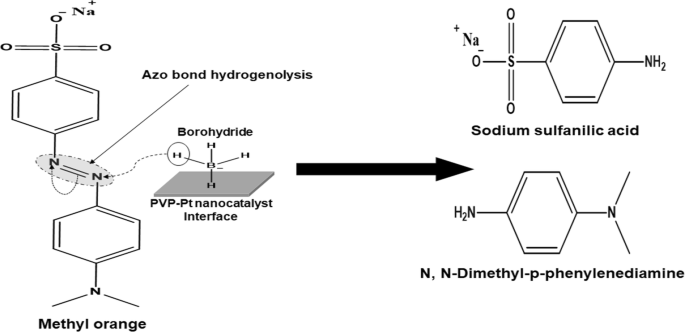
Fabrication of palladium and platinum nanocatalysts stabilized by polyvinylpyrrolidone and their use in the hydrogenolysis of methyl orange | SpringerLink

Effective Catalytic Reduction of Methyl Orange Catalyzed by the Encapsulated Random Alloy Palladium‐Gold Nanoparticles Dendrimer. - Ilunga - 2017 - ChemistrySelect - Wiley Online Library



